
40 Difference Between Diffusion And Osmosis Laboratory Hub The difference in concentration of a material between two regions is referred to as a concentration gradient. diffusion and osmosis take place along concentration gradients, with nanoparticles moving from regions with greater concentrations to lower concentration areas. Get the definition and examples of osmosis and diffusion. learn the differences between osmosis and diffusion and how solute and solvent particles behave.

4 3 Diffusion And Osmosis Lab Pdf Osmosis Chemistry What is the difference between a semi permeable and a selectively permeable membrane part 2. plasmolysis—observing osmosis in a living system, elodea if a plant cell is immersed in a solution that has a higher solute concentration than that of the cell, water will leave enter (circle one) the cell. Explore the key differences between diffusion and osmosis: process mechanisms, substance involvement, energy requirements, and biological roles. Table of contents part 1: brownian motion materials procedure lab questions part 2: diffusion across a semipermeable membrane materials procedure data lab questions part 3: osmosis and the cell membrane prediction materials procedure results lab questions part 4: experimental design materials design contributors and attributions the cell membrane plays the dual roles of protecting the living. Diffusion and osmosis table of contents diffusion diffusion is the net movement of a substance from high concentration to low concentration. this difference in concentration is referred to as a concentration gradient. this movement does not require any external energy, but uses the free energy intrinsic to the system.
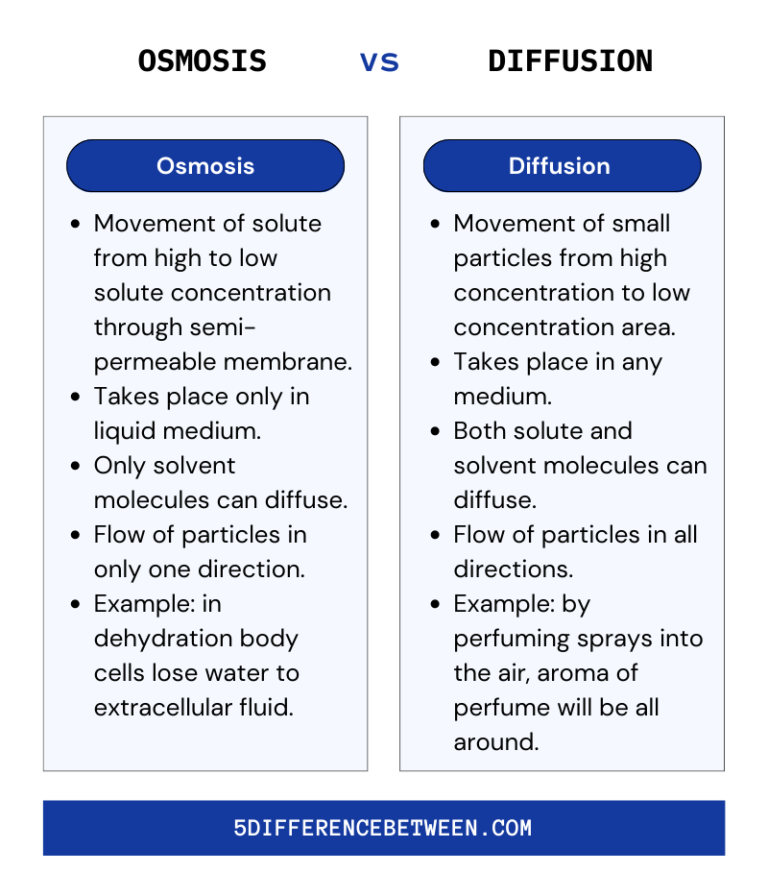
5 Difference Between Osmosis And Diffusion Osmosis Vs Diffusion Table of contents part 1: brownian motion materials procedure lab questions part 2: diffusion across a semipermeable membrane materials procedure data lab questions part 3: osmosis and the cell membrane prediction materials procedure results lab questions part 4: experimental design materials design contributors and attributions the cell membrane plays the dual roles of protecting the living. Diffusion and osmosis table of contents diffusion diffusion is the net movement of a substance from high concentration to low concentration. this difference in concentration is referred to as a concentration gradient. this movement does not require any external energy, but uses the free energy intrinsic to the system. The diffusion rate can be increased by shaking or mixing the solution to increase the velocity of the molecules being combined. when water molecules move across a semi permeable membrane due to a difference in concentration, the process is called osmosis. 3. when you have completed all the questions, upload this document to the lab report assignment in canvas. virtual lab activities activity osmosis in potatoes 1. what is the difference between osmosis and diffusion? osmosis is when water moves through a membrane from an area with less stuff dissolved in it to an area with more stuff dissolved.
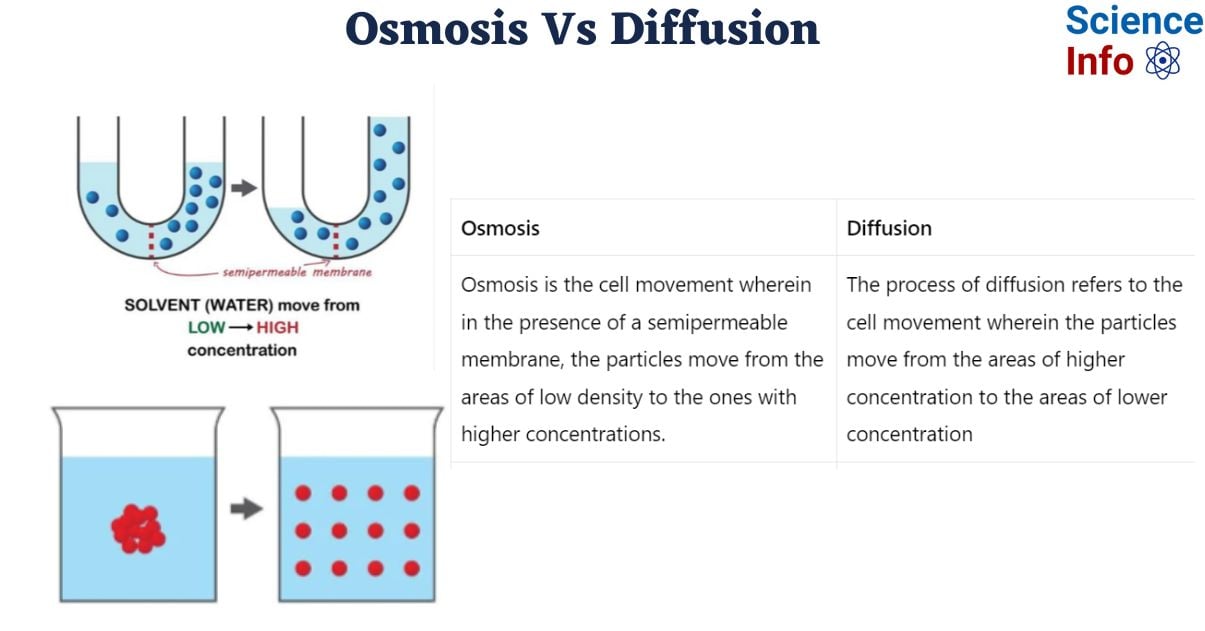
Difference Between Osmosis And Diffusion The diffusion rate can be increased by shaking or mixing the solution to increase the velocity of the molecules being combined. when water molecules move across a semi permeable membrane due to a difference in concentration, the process is called osmosis. 3. when you have completed all the questions, upload this document to the lab report assignment in canvas. virtual lab activities activity osmosis in potatoes 1. what is the difference between osmosis and diffusion? osmosis is when water moves through a membrane from an area with less stuff dissolved in it to an area with more stuff dissolved.
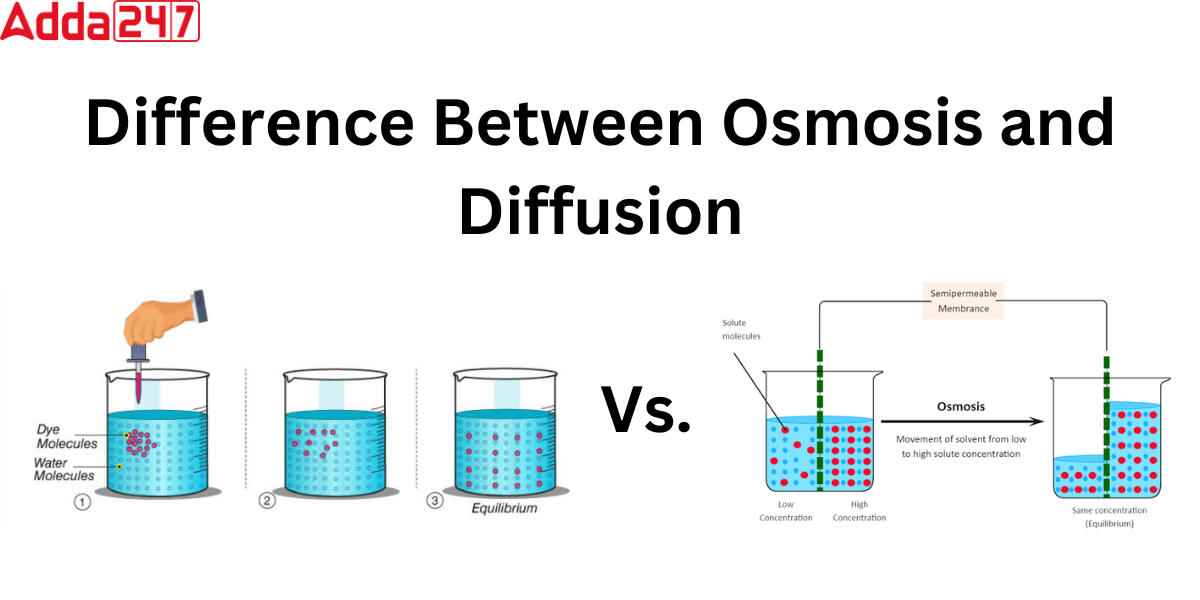
Difference Between Osmosis And Diffusion For Class 9
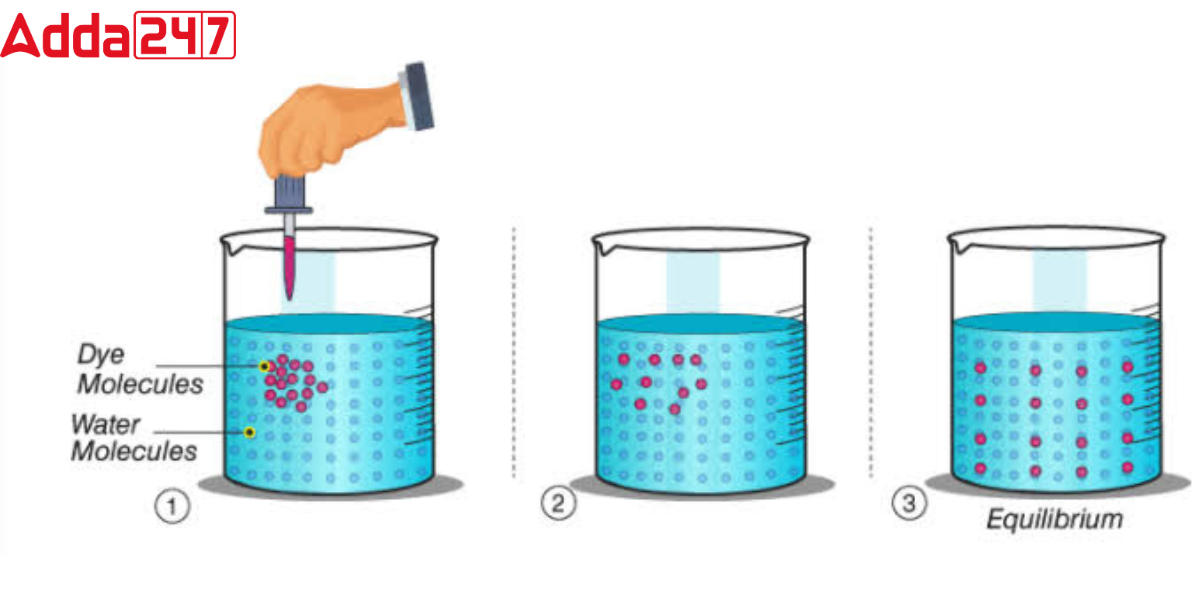
Difference Between Osmosis And Diffusion For Class 9