
Solutions Table Pdf The table is completed with the help of key equations representing the relationship between [h3o⁺], [oh⁻], and ph. the identified solutions are classified as acidic, basic, or neutral. Science chemistry chemistry questions and answers complete the following table. (all solutions are at 25 ∘c.) [h3o ] [oh−] ph acidic or.

тпйsolved Complete The Following Table All Solutions Are At 25 тиш C Free expert solution to complete the following table. (all solutions are at 25 ∘c.) [h3o ] [oh−] ph acidic or basic. To complete the table, we need to use the following formulas: ph = log [h3o ] poh = log [oh ] ph poh = 14 (at 25°c) if ph < 7, the solution is acidic. if ph > 7, the continue reading ask a new question discover more from: chemistry iichem1050 university of guelph humber 62documents go to course 10 lab 4 lab report chemistry ii100% (6) 10. Complete the table. (all solutions are at 25 °c.) tro chemistry: a molecular approach 4th edition solution to problem 51 in chapter 16. The table requires calculating concentrations and ph based on the relationships between hydronium ion concentration, hydroxide ion concentration, ph, and whether each solution is acidic or basic at 25°c. the calculations show solution 1 is acidic, while solutions 2, 3, and 4 are basic. the missing values have been filled in using established scientific formulas.
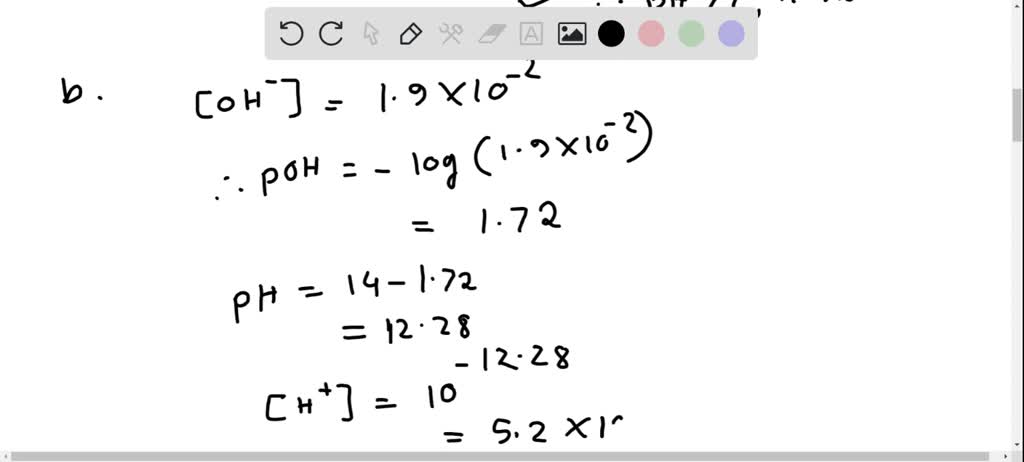
тпйsolved Complete The Following Table All Solutions Are At 25 тиш C Complete the table. (all solutions are at 25 °c.) tro chemistry: a molecular approach 4th edition solution to problem 51 in chapter 16. The table requires calculating concentrations and ph based on the relationships between hydronium ion concentration, hydroxide ion concentration, ph, and whether each solution is acidic or basic at 25°c. the calculations show solution 1 is acidic, while solutions 2, 3, and 4 are basic. the missing values have been filled in using established scientific formulas. To complete the table provided, we need to calculate the missing values for [oh ], ph, and whether the solution is acidic or basic based on the given [h3o ] values. A a basic solution has an h3o* concentration greater than 1.0 x 10 7 m and a ph value lower than 7.0. b a basic solution has an oh concentration greater than 1.0 x 10 7 m and a ph value lower than 7.0. a basic solution has an h3o* concentration greater than 1.0 x 10 7 m and a ph value greater than 7.0.

Solved Complete The Following Table All Solutions Are At 25 Chegg To complete the table provided, we need to calculate the missing values for [oh ], ph, and whether the solution is acidic or basic based on the given [h3o ] values. A a basic solution has an h3o* concentration greater than 1.0 x 10 7 m and a ph value lower than 7.0. b a basic solution has an oh concentration greater than 1.0 x 10 7 m and a ph value lower than 7.0. a basic solution has an h3o* concentration greater than 1.0 x 10 7 m and a ph value greater than 7.0.

Solved Complete The Following Table All Solutions Are At Chegg