
Difference Between Gas And Vapor Gas Vs Vapor The important difference of a vapor and a gas essentially, when compressed (increase in pressure) the vapor will condense the gas will convert in a critical fluid!. The differences between vapor and gas are very few and it is essential to know the key differences between the two to avoid any kind of confusion that may occur. knowing the difference between the two can help one differentiate the meaning and difference of one over the other. vapour is simply defined as the phase of a gas at a temperature where the same substance can exist in both liquid and.
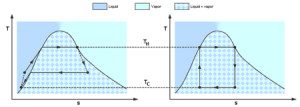
Difference Between Vapor And Gas Difference Between A gas would be most vapor like right at a boundary with a condensed state. the further a state is from a transition between a condensed state and gas state, the less vapor like it'd be. Key differences between vapor and gas generally, the vapor phase includes two distinct substances at room temperature, while the gas phase involves a single substance within a specific thermodynamic range at the same temperature. this forms the primary distinction between vapor and gas. the table below further elaborates on these differences. Gas vs. vapor what's the difference? gas and vapor are both forms of matter that exist in a gaseous state. however, there are some key differences between the two. gas refers to a substance that is in its gaseous state at room temperature and atmospheric pressure, such as oxygen or nitrogen. on the other hand, vapor refers to the gaseous form of a substance that is typically a liquid or solid. A gas refers to a substance that has a single defined thermodynamic state at room temperature whereas a vapor refers to a substance that is a mixture of two phases at room temperature, namely gaseous and liquid phase.

Gas Vs Vapor Difference Between Gas vs. vapor what's the difference? gas and vapor are both forms of matter that exist in a gaseous state. however, there are some key differences between the two. gas refers to a substance that is in its gaseous state at room temperature and atmospheric pressure, such as oxygen or nitrogen. on the other hand, vapor refers to the gaseous form of a substance that is typically a liquid or solid. A gas refers to a substance that has a single defined thermodynamic state at room temperature whereas a vapor refers to a substance that is a mixture of two phases at room temperature, namely gaseous and liquid phase. The vapor phase of substance is called gas when it is above critical temperature. whereas vapor phase implies that the current phase is not far from a state of condensation [that is the vapor can easily get converted into liquid phase]. Difference between vapor and a gas thermodynamics class 49 lesson with certificate for engineering courses.

Difference Between Gas And Vapor Gas Vs Vapor The vapor phase of substance is called gas when it is above critical temperature. whereas vapor phase implies that the current phase is not far from a state of condensation [that is the vapor can easily get converted into liquid phase]. Difference between vapor and a gas thermodynamics class 49 lesson with certificate for engineering courses.