
Osmosis Vs Diffusion Understanding The Key Differences Get the definition and examples of osmosis and diffusion. learn the differences between osmosis and diffusion and how solute and solvent particles behave. The main difference between osmosis and diffusion is that osmosis moves water across a membrane, while diffusion spreads out solutes in a space.
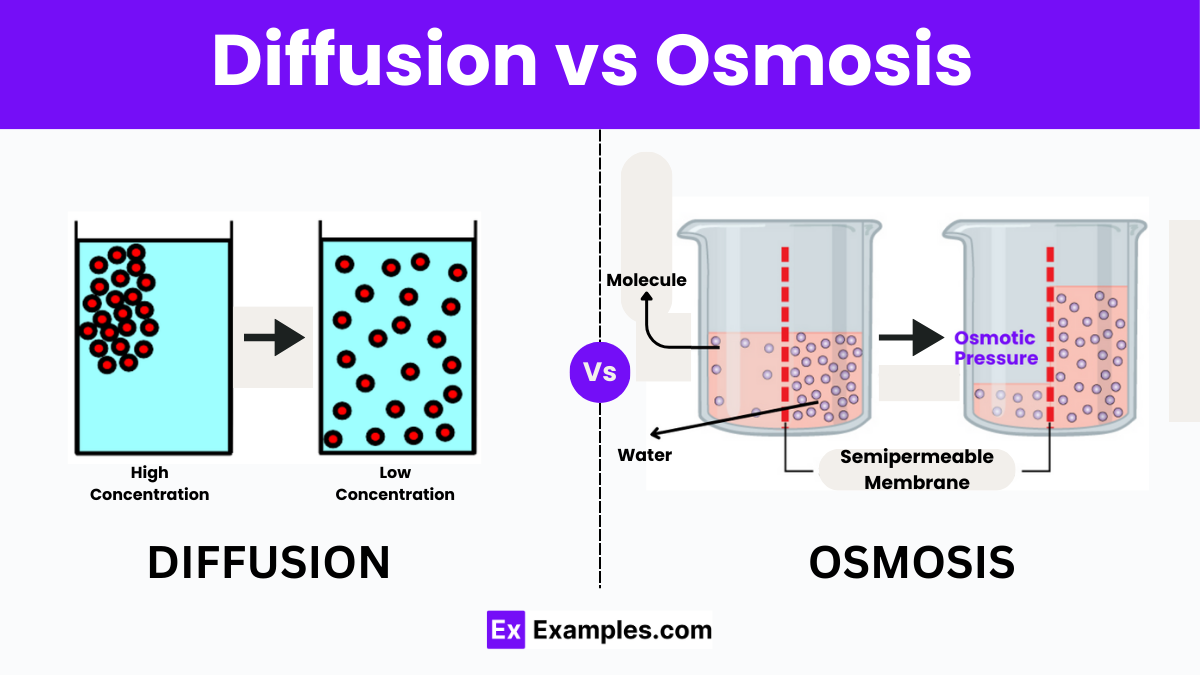
Diffusion Vs Osmosis Differences Explained Understanding diffusion vs osmosis: key differences explained with real life examples ellieb ever wondered how a drop of ink disperses in water, or why plants don’t wilt when well watered? these aren’t magic tricks but natural phenomena known as diffusion and osmosis. while they might seem similar at first glance, they’re actually quite distinct. in the intriguing area of biology, these. In the study of biology, understanding the movement of molecules and ions across cellular membranes is fundamental. two essential processes that facilitate this movement are osmosis and diffusion. while they are related and often occur simultaneously within biological systems, they are distinct mechanisms with unique characteristics and roles. Distance: osmosis occurs more quickly the closer the distance is between the two sides of the membrane. understanding diffusion: 8 key points to know the movement of particles (atoms, molecules, ions, etc.) from a region of higher concentration to an area of lower concentration is known as diffusion, which is a sort of passive transport. The main difference between diffusion and osmosis is that diffusion is the movement of particles from high to low concentration, while osmosis is the movement of solvent molecules to equalize concentration through a semipermeable membrane.
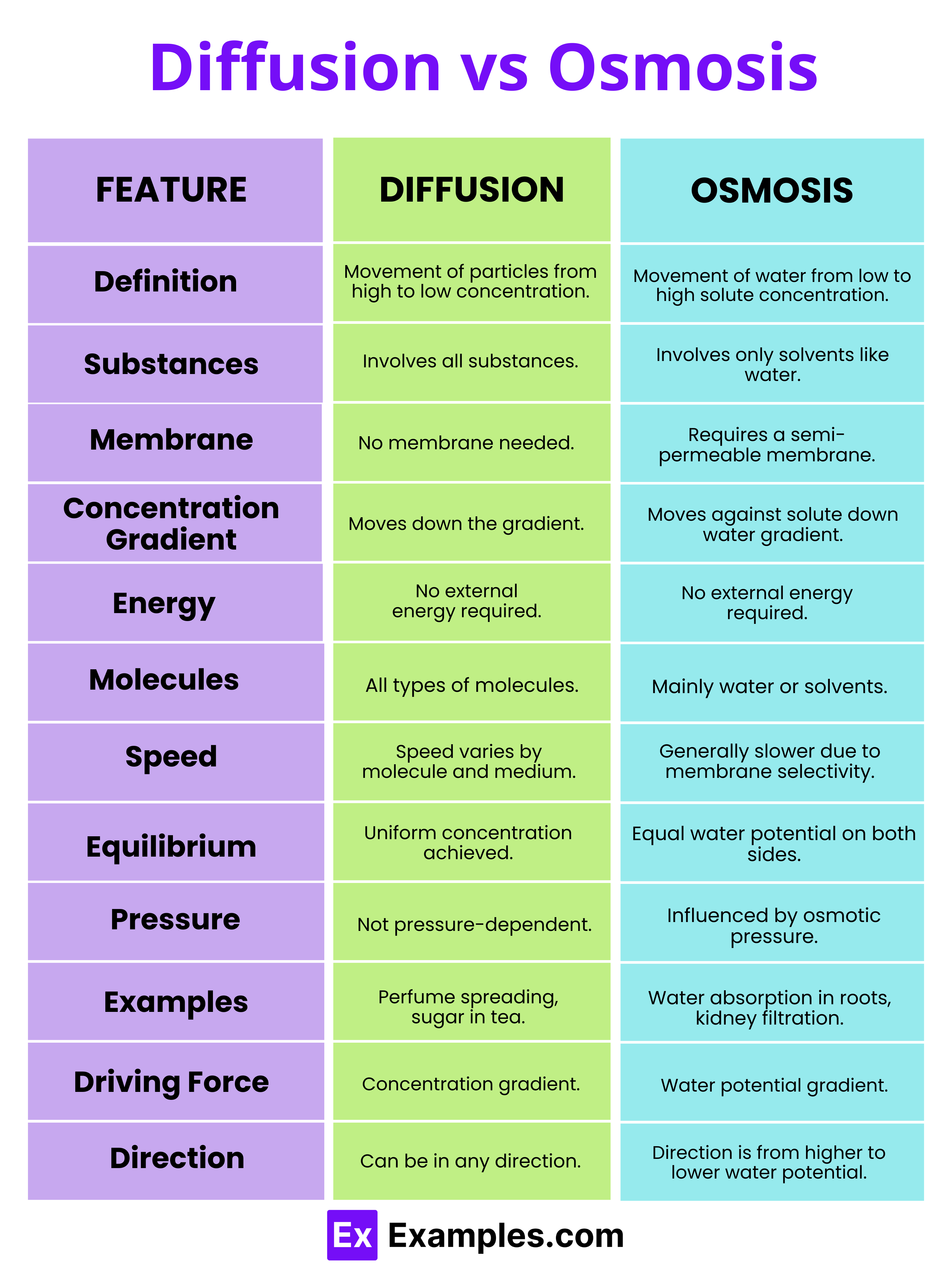
Diffusion Vs Osmosis Differences Explained Distance: osmosis occurs more quickly the closer the distance is between the two sides of the membrane. understanding diffusion: 8 key points to know the movement of particles (atoms, molecules, ions, etc.) from a region of higher concentration to an area of lower concentration is known as diffusion, which is a sort of passive transport. The main difference between diffusion and osmosis is that diffusion is the movement of particles from high to low concentration, while osmosis is the movement of solvent molecules to equalize concentration through a semipermeable membrane. Process of osmosis vs. diffusion diffusion occurs when the spontaneous net movement of particles or molecules spreads them from an area of high concentration to an area of low concentration through a semipermeable membrane. it is simply the statistical outcome of random motion. as time progresses, the differential gradient of concentrations between high and low will drop (become increasingly. Diffusion vs osmosis – differences explained diffusion and osmosis are two fundamental processes that facilitate the movement of particles in biological systems, playing crucial roles in maintaining the homeostasis of cells and organisms. despite their similar nature in facilitating the transport of substances, they have distinct characteristics and functions. understanding the differences.

Osmosis Vs Diffusion Definition And Examples 40 Off Process of osmosis vs. diffusion diffusion occurs when the spontaneous net movement of particles or molecules spreads them from an area of high concentration to an area of low concentration through a semipermeable membrane. it is simply the statistical outcome of random motion. as time progresses, the differential gradient of concentrations between high and low will drop (become increasingly. Diffusion vs osmosis – differences explained diffusion and osmosis are two fundamental processes that facilitate the movement of particles in biological systems, playing crucial roles in maintaining the homeostasis of cells and organisms. despite their similar nature in facilitating the transport of substances, they have distinct characteristics and functions. understanding the differences.