
Enthalpy Vs Entropy Pdf Enthalpy Entropy We investigate enthalpy entropy compensation for melting of nearest neighbor doublets in dna. enthalpy: enthalpy is defined as the total heat content or total useful energy of a substance. but they have the same unit joule kelvin like work & energy. entropy change is the amount of energy dispersed reversibly at a specific temperature. Enthalpy and entropy are important concepts in thermodynamics that relate heat and work. enthalpy can be defined as the total energy in a system, whereas entropy is defined as the thermal energy of a system per unit temperature.

Enthalpy Vs Entropy Maingolf Here, t is the absolute temperature, ∆h is the change in enthalpy, and ∆s is the change in entropy. according to this equation, an increase in the enthalpy of a system causes an increase in its entropy. in chemistry, thermodynamics refers to the field that deals with heat and energy of a system and the study of energy change of a system. Enthalpy thermodynamics is the study of the relationship between heat (or energy) and work. enthalpy is a central factor in thermodynamics. it is the heat content of a system. the heat that passes into or out of the system during a reaction is the enthalpy change. whether the enthalpy of the system increases (i.e. when energy is added) or decreases (because energy is given off) is a crucial. Enthalpy vs entropy the main difference between enthalpy and entropy is that enthalpy is a measurement of a system's total energy, which is equal to the sum of internal energy plus the product of pressure and volume. entropy, on the other hand, is the amount of thermal energy in a system that cannot be converted into work. Enthalpy is a thermodynamic property that represents the total heat content of a system, including both internal energy and the energy associated with pressure and volume, while entropy is a thermodynamic property that measures the level of disorder or randomness in a system.
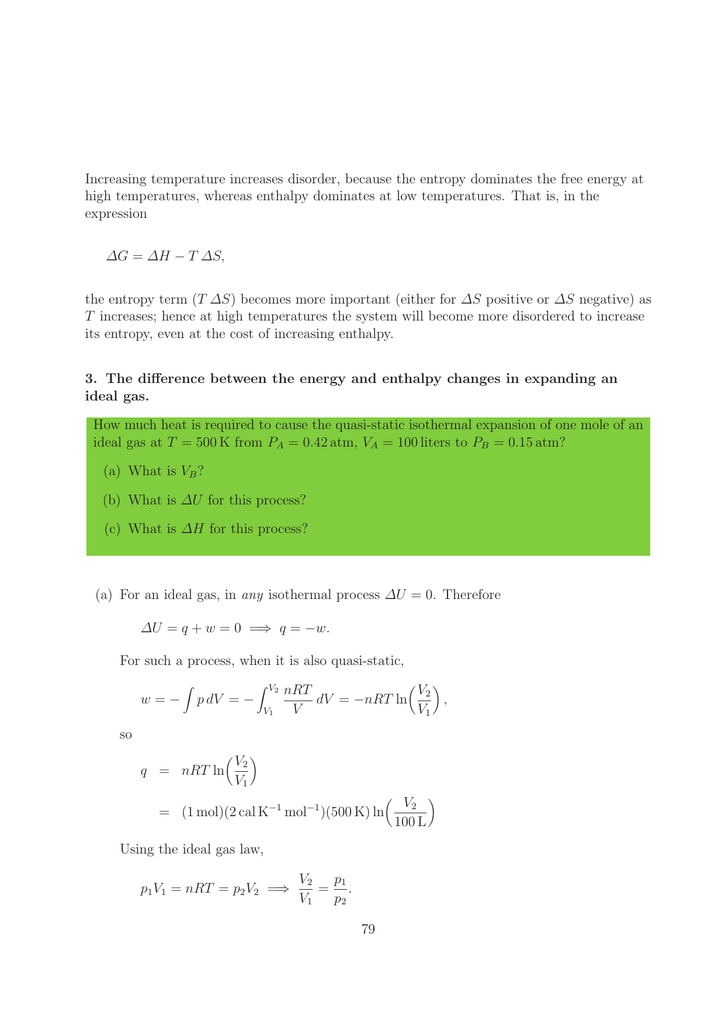
Enthalpy Vs Entropy Maingolf Enthalpy vs entropy the main difference between enthalpy and entropy is that enthalpy is a measurement of a system's total energy, which is equal to the sum of internal energy plus the product of pressure and volume. entropy, on the other hand, is the amount of thermal energy in a system that cannot be converted into work. Enthalpy is a thermodynamic property that represents the total heat content of a system, including both internal energy and the energy associated with pressure and volume, while entropy is a thermodynamic property that measures the level of disorder or randomness in a system. Enthalpy vs. entropy what's the difference? enthalpy and entropy are both thermodynamic properties that describe different aspects of a system. enthalpy, denoted as h, is a measure of the total energy of a system, including both its internal energy and the energy associated with pressure and volume. Understand entropy vs. enthalpy, their calculations, and their importance in chemical reactions and energy systems.

Enthalpy Vs Entropy Know The Difference Enthalpy vs. entropy what's the difference? enthalpy and entropy are both thermodynamic properties that describe different aspects of a system. enthalpy, denoted as h, is a measure of the total energy of a system, including both its internal energy and the energy associated with pressure and volume. Understand entropy vs. enthalpy, their calculations, and their importance in chemical reactions and energy systems.
Enthalpy Vs Entropy The Science Behind Energy And Disorder