
Lesson 1 Atomic Theory Pdf This document provides a timeline of the development of atomic models from ancient greek philosophers to present day. it describes early theories from democritus and aristotle that matter is made of indivisible particles. john dalton later proposed atoms as indestructible, indivisible spheres that combine in simple ratios. j.j. thomson's discovery of electrons led to the "plum pudding" model. 1 atomic theory the movers and shakers of the subatomic world. 2 dalton • in the early 1800’s, the english chemist john dalton did a number of experiments that eventually led to the acceptance of the idea of atoms 3 dalton’s atomic theory • all elements are composed of atoms. atoms are invisible and indestructible particles. 4 continued.
Atomic Theory Ppt Final Complete Grade 9 Lesson Pptx The development of atomic theory do theories in science stay the same? • ideas and theories in science change as new information is gathered. (question 1) our theory about the atom has changed over time as new studies are done. Greek philosophers democritus and aristotle debated early atomic theory, with democritus correctly proposing atoms as indivisible units but aristotle rejecting this view. in 1803, john dalton established the modern atomic theory, though some aspects were later disproven. over subsequent decades, discoveries like the electron, proton, neutron and quantum nature of electrons led to developments. History of atomic theory. democritus 460 371 b.c. ancient greek philosopher believed all matter consisted of extremely small particles that could not be divided atoms, from greek word atomos, means “uncut” or “indivisible” aristotle. The document outlines the development of atomic theories from democritus to schrodinger. it describes early atomic models proposed by democritus, empedocles, and aristotle. it then discusses seminal contributions from dalton, goldstein, thomson, rutherford, bohr, chadwick, and schrodinger that led to modern atomic theory, including dalton's atomic theory, thomson's plum pudding model.

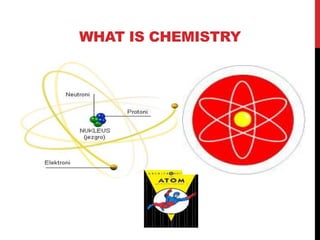
No Prep Learning Atomic Theory History Teacher Ppt Guided Notes History of atomic theory. democritus 460 371 b.c. ancient greek philosopher believed all matter consisted of extremely small particles that could not be divided atoms, from greek word atomos, means “uncut” or “indivisible” aristotle. The document outlines the development of atomic theories from democritus to schrodinger. it describes early atomic models proposed by democritus, empedocles, and aristotle. it then discusses seminal contributions from dalton, goldstein, thomson, rutherford, bohr, chadwick, and schrodinger that led to modern atomic theory, including dalton's atomic theory, thomson's plum pudding model. Democritus ( bc) was the first person to come up with the idea of atom believed that all matter was composed of indivisible particles he called “atoms” which is derived from the greek word “atomos” – meaning indivisible he also believed that different atoms: are different sizes have different properties other philosophers of that time did not agree with his theories. Carbon 12 has 12 protons and 12 neutrons… therefore, defining one atomic mass unit as 1 12 of a carbon 12 atom, means that 1 proton has a mass of 1 amu and 1 neutron has a mass of 1 amu. isotopes we now know that a number of the postulates of dalton’s atomic theory were wrong. we know that particles smaller than the atom exist.

Introduction To Atomic Theory Ppt Democritus ( bc) was the first person to come up with the idea of atom believed that all matter was composed of indivisible particles he called “atoms” which is derived from the greek word “atomos” – meaning indivisible he also believed that different atoms: are different sizes have different properties other philosophers of that time did not agree with his theories. Carbon 12 has 12 protons and 12 neutrons… therefore, defining one atomic mass unit as 1 12 of a carbon 12 atom, means that 1 proton has a mass of 1 amu and 1 neutron has a mass of 1 amu. isotopes we now know that a number of the postulates of dalton’s atomic theory were wrong. we know that particles smaller than the atom exist.