
Flt3 Mutations In Acute Myeloid Leukemia Aml Biorender Science Treatment options for people with the aggressive blood cancer acute myeloid leukemia (aml) have expanded yet again with a new approval from the food and drug administration (fda). on july 20, the agency approved quizartinib (vanflyta) combined with chemotherapy as part of the initial treatment of people with aml that has a specific change in a gene called flt3. genetic changes in flt3 are. Flt3 mutation is commonly present in newly diagnosed patients with acute myeloid leukemia, and confers high relapse risk. targeted therapies with flt3 inhibitors improve survival when used as a single agent in a salvage setting, and more so when.
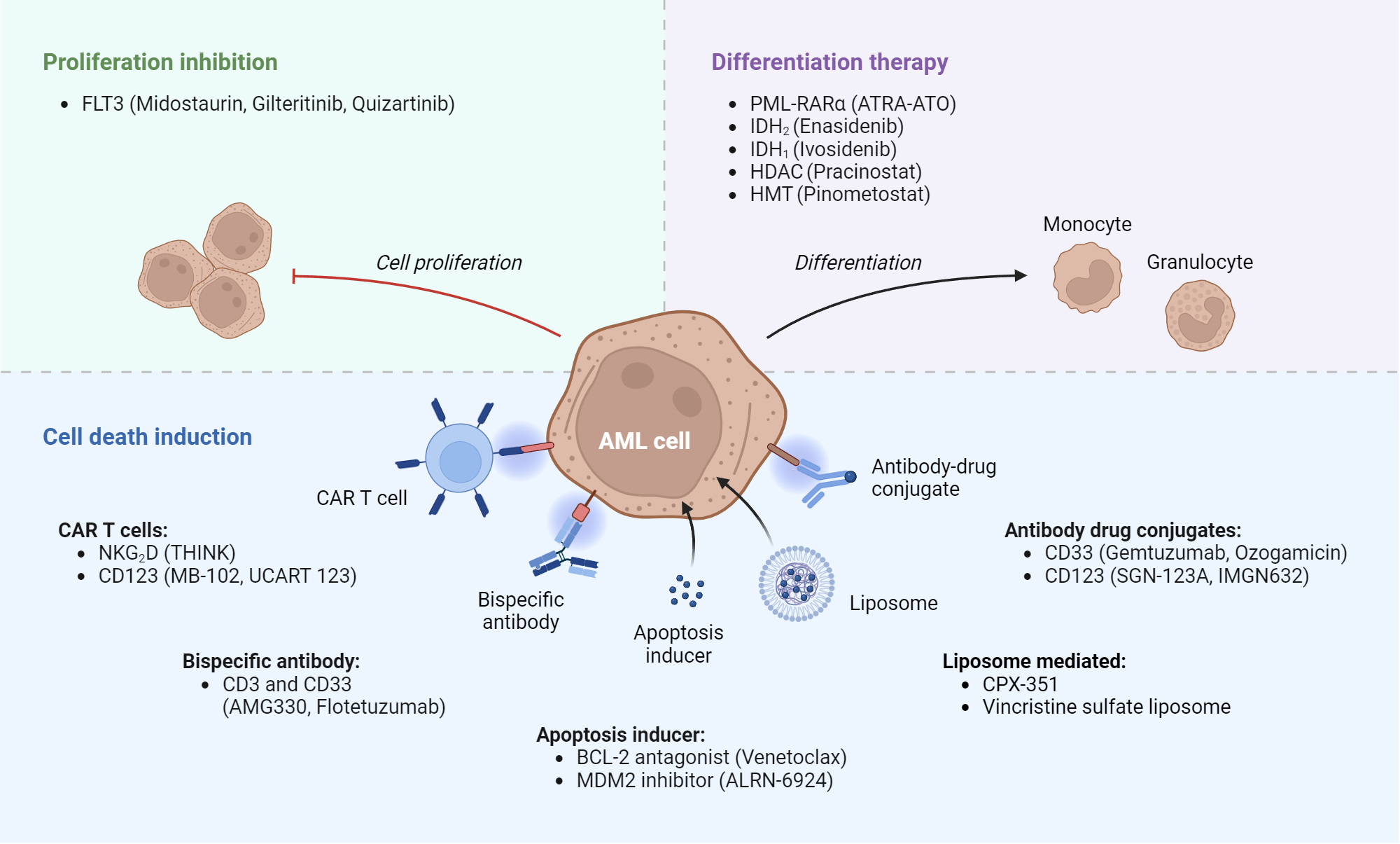
Acute Myeloid Leukemia Targets And Therapies Biorender Science Templates Rye brook, n.y., july 25, 2023 – the u.s. food and drug administration (fda) last week approved quizartinib (vanflyta®) in combination with standard chemotherapies for the treatment of adult patients with newly diagnosed acute myeloid leukemia (aml) that carries a genetic mutation called flt3 itd. quizartinib is the first drug designed to target the flt3 itd mutation, which accounts for. The understanding of the molecular pathobiology of acute myeloid leukemia (aml) has spurred the identification of therapeutic targets and the development of corresponding novel targeted therapies. The acute myeloid leukemia (aml) treatment landscape has changed substantially since 2017. new targeted drugs have emerged, including venetoclax to target b cell lymphoma 2, midostaurin and gilteritinib to target flt3, and ivosidenib and enasidenib to target mutant isocitrate dehydrogenase 1 and 2, respectively. other additions include reapproval of gemtuzumab ozogomycin to target cd33. Flt3 mutations are present in approximately 25% to 30% of acute myeloid leukemia (aml) cases, leading to aggressive disease and poor outcomes. 1 flt3 inhibitors encompass a diverse group of agents that target mutations in the flt3 gene, which are implicated in the development of aml (online table).
Acute Myeloid Leukemia Plos One The acute myeloid leukemia (aml) treatment landscape has changed substantially since 2017. new targeted drugs have emerged, including venetoclax to target b cell lymphoma 2, midostaurin and gilteritinib to target flt3, and ivosidenib and enasidenib to target mutant isocitrate dehydrogenase 1 and 2, respectively. other additions include reapproval of gemtuzumab ozogomycin to target cd33. Flt3 mutations are present in approximately 25% to 30% of acute myeloid leukemia (aml) cases, leading to aggressive disease and poor outcomes. 1 flt3 inhibitors encompass a diverse group of agents that target mutations in the flt3 gene, which are implicated in the development of aml (online table). The treatment landscape for acute myeloid leukemia (aml) is evolving rapidly, as research discoveries at sylvester comprehensive cancer center at the university of miami miller school of medicine. Purpose of review this review seeks to identify and describe novel genetic and protein targets and their associated therapeutics currently being used or studied in the treatment of acute myeloid leukemia (aml). recent findings over the course of the last 5–6 years, several targeted therapies have been approved by the fda, for the treatment of both newly diagnosed as well as relapsed.

Case Study Presents 3 Potential Novel Targets In Acute Myeloid Leukemia The treatment landscape for acute myeloid leukemia (aml) is evolving rapidly, as research discoveries at sylvester comprehensive cancer center at the university of miami miller school of medicine. Purpose of review this review seeks to identify and describe novel genetic and protein targets and their associated therapeutics currently being used or studied in the treatment of acute myeloid leukemia (aml). recent findings over the course of the last 5–6 years, several targeted therapies have been approved by the fda, for the treatment of both newly diagnosed as well as relapsed.