
Osmosis Vs Diffusion Understanding The Key Differences Get the definition and examples of osmosis and diffusion. learn the differences between osmosis and diffusion and how solute and solvent particles behave. Diffusion involves the movement of molecules from an area of higher concentration to an area of lower concentration, while osmosis specifically refers to the movement of water molecules across a semi permeable membrane. in diffusion, any type of molecule can diffuse, whereas in osmosis, only water molecules are involved. diffusion occurs in both liquids and gases, while osmosis primarily.

Osmosis Vs Diffusion Definition And Examples Artofit Osmosis and diffusion both happen when there’s more of a substance in one place, and less of it in an adjacent place. the movement is caused by the natural tendency of things to spread out evenly until the concentrations are equalized. and the greater the difference in concentrations, the faster the movement. Osmosis is a biophysical process occurring commonly in biological systems where solvent molecules move across a semi permeable membrane towards a region of high solute concentration. Osmosis vs. diffusion is misleading as far as titles go. both are kinds of passive transport. passive transport is the gradual movement of molecules from one concentration to another until they are equalized, or at least that’s the shortest definition. osmosis and diffusion are two ways to accomplish this equilibrium. Explore the key differences between diffusion and osmosis: process mechanisms, substance involvement, energy requirements, and biological roles.

Osmosis Vs Diffusion Definition And Examples Artofit Osmosis vs. diffusion is misleading as far as titles go. both are kinds of passive transport. passive transport is the gradual movement of molecules from one concentration to another until they are equalized, or at least that’s the shortest definition. osmosis and diffusion are two ways to accomplish this equilibrium. Explore the key differences between diffusion and osmosis: process mechanisms, substance involvement, energy requirements, and biological roles. Conclusion diffusion and osmosis are fundamental processes that play vital roles in biological systems. while diffusion involves the movement of particles along a concentration gradient, osmosis specifically refers to the movement of water molecules across a selectively permeable membrane. The two main types of osmosis are endosmosis and exosmosis. the movements in osmosis seek to equalize solvent concentration, although it does not achieve this. examples of osmosis an example of osmosis is the movement of water into root hair cells. swelling of red blood cells when placed in freshwater biological importance of osmosis.
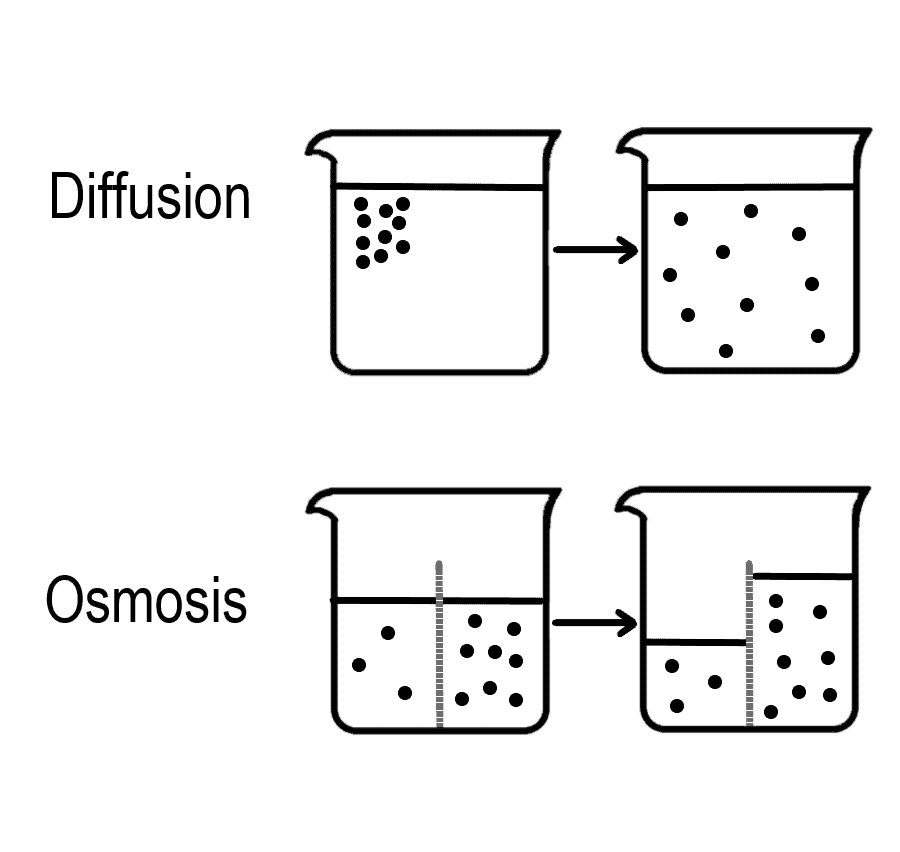
Osmosis Vs Diffusion Definition And Examples 57 Off Conclusion diffusion and osmosis are fundamental processes that play vital roles in biological systems. while diffusion involves the movement of particles along a concentration gradient, osmosis specifically refers to the movement of water molecules across a selectively permeable membrane. The two main types of osmosis are endosmosis and exosmosis. the movements in osmosis seek to equalize solvent concentration, although it does not achieve this. examples of osmosis an example of osmosis is the movement of water into root hair cells. swelling of red blood cells when placed in freshwater biological importance of osmosis.