
Process Validation Guidance Fda Presentation Pdf Audit Food And A paradigm shift is underway in process validation. the fda revised the guidance to industry for process validation in january 2011. this guidance defines process validation as “the collection and evaluation of data, from the process design stage through commercial production which establishes. The 2011 guidance revises and replaces fda’s guidance for industry entitled guideline on general principles of process validation, issued in may 1987 (the 1987 guideline).

Process Validation Guidance Pdf Verification And Validation Food The authors desribe the three stage approach to validation that is outlined in the new guidance and discuss questions surrounding implementation. fda's 2011 process validation: general principles and practices guidance created a systemic shift in industry's approaches to validation programs. the. In 2011, the us food and drug administration (fda) introduced the revised “guidance for industry: process validation: general principles and practices.” the document incorporated principles from existing ich guidance in place since 2005 (ich q8 and q9) and 2008 (ich q10). , , ispe formed their product quality lifecycle implementation (pqli)® initiative to provide guidance on the practical. A paradigm shift is underway in process validation. the fda revised the guidance to industry for process validation in january 2011. this guidance defines process validation as “the collection and evaluation of data, from the process design stage through commercial production which establishes scientific evidence that a process is capable of consistently delivering quality product.” the. This article has been written as a contribution to the industry's efforts in migrating from a document driven to a data driven compliance mindset. a combination of target product profile, control engineering, and general sum principle techniques is presented as the basis of a simple but scalable lifecycle compliance model in support of modernized process validation. unit operations and.

Understanding The New Fda Process Validation Guidelines A Shift From A paradigm shift is underway in process validation. the fda revised the guidance to industry for process validation in january 2011. this guidance defines process validation as “the collection and evaluation of data, from the process design stage through commercial production which establishes scientific evidence that a process is capable of consistently delivering quality product.” the. This article has been written as a contribution to the industry's efforts in migrating from a document driven to a data driven compliance mindset. a combination of target product profile, control engineering, and general sum principle techniques is presented as the basis of a simple but scalable lifecycle compliance model in support of modernized process validation. unit operations and. The fda definition for process validation is " the collection and evaluation of data, from the process design stage through commercial production , which establishes scientific evidence that a. Fda’s definition – 2011 “for purposes of this guidance, process validation is defined as the collection and evaluation of data , from the process design stage through commercial production, which establishes scientific evidence that a process is capable of consistently delivering quality product.

Fda Lifecycle Approach To Process Validation What Why How Pdf The fda definition for process validation is " the collection and evaluation of data, from the process design stage through commercial production , which establishes scientific evidence that a. Fda’s definition – 2011 “for purposes of this guidance, process validation is defined as the collection and evaluation of data , from the process design stage through commercial production, which establishes scientific evidence that a process is capable of consistently delivering quality product.
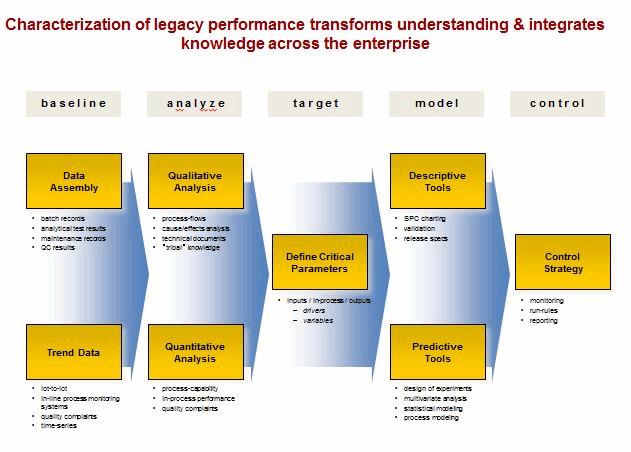
Overcoming The Challenge Of The 2011 Fda Process Validation Guidance

Overcoming The Challenge Of The 2011 Fda Process Validation Guidance
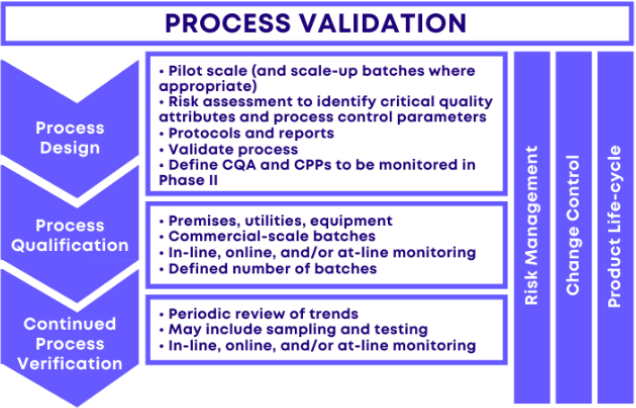
Stages And Types Of Process Validation Fda Guidance On 52 Off