
Ich Q7 Pdf Quality Management System Verification And Validation Q7 good manufacturing practice guidance for active pharmaceutical ingredients guidance for industry1 this guidance represents the current thinking of the food and drug administration (fda or. Process validation and ich q7 u.s. food and drug administration 172k subscribers subscribe.

Gmp Ich Q7 Document Kit Pdf Verification And Validation Quality Process validation pda pic s ich q7 training pic s and parenteral drug association (pda), september 2014 reproduction prohibited for commercial purposes. reproduction for internal use is authorised, provided that the source is acknowledged. This would include the validation of critical process steps determined to impact the quality of the api. however, it should be noted that the fact that a company chooses to validate a process step does not necessarily define that step as critical. the guidance in this document would normally be applied to the steps shown in gray in table 1. This would include the validation of critical process steps determined to impact the quality of the api. however, it should be noted that the fact that a company chooses to validate a process step does not necessarily define that step as critical. The ich q7 guidelines provide guidance on these processes, including requirements for process validation, process controls, and testing of intermediate and finished products.
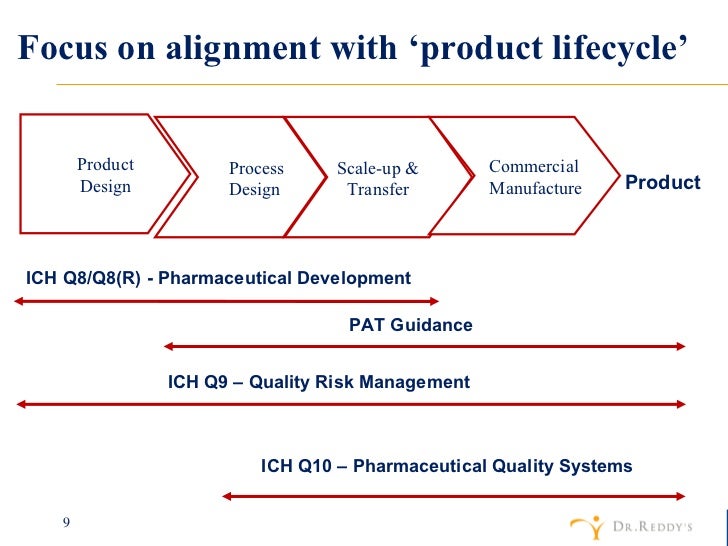
Revised Process Validation This would include the validation of critical process steps determined to impact the quality of the api. however, it should be noted that the fact that a company chooses to validate a process step does not necessarily define that step as critical. The ich q7 guidelines provide guidance on these processes, including requirements for process validation, process controls, and testing of intermediate and finished products. Process validation for apis is discussed in the fda ich guidance for industry, q7 good manufacturing practice guidance for active pharmaceutical ingredients (ich q7), available on the internet at. 1. introduction (background) process validation can be defined as documented evidence that the process, operated within established parameters, can perform effectively and reproducibly to produce a medicinal product meeting its predetermined specifications and quality attributes (ich q7). continuous process verification has been introduced to cover an alternative approach to process validation.
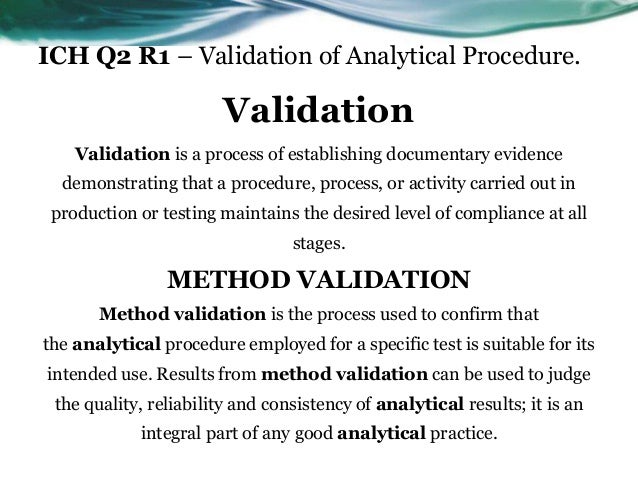
Ich Q2 Analytical Method Validation Process validation for apis is discussed in the fda ich guidance for industry, q7 good manufacturing practice guidance for active pharmaceutical ingredients (ich q7), available on the internet at. 1. introduction (background) process validation can be defined as documented evidence that the process, operated within established parameters, can perform effectively and reproducibly to produce a medicinal product meeting its predetermined specifications and quality attributes (ich q7). continuous process verification has been introduced to cover an alternative approach to process validation.