
Solved Complete The Following Table All Solutions Are At Chegg To start filling in the first blank in the table, use the given ph value of 3.15 to find the concentration of [h 3 o ] by using the formula p h = − log [h ]. To complete the given table, we first need to understand the relationship between [h3o⁺], [oh⁻], ph, and whether the solution is acidic or basic. this can be summarized using the following key equations:.
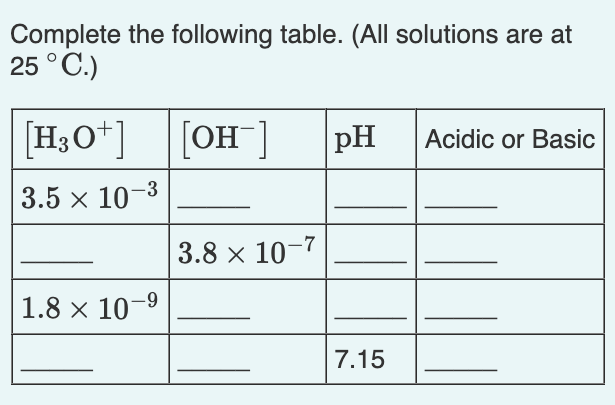
Solved Complete The Following Table All Solutions Are Chegg Complete the table. (all solutions are at 25 °c.) tro chemistry: a molecular approach 4th edition solution to problem 51 in chapter 16. Video answer: in this question. oh you have to complete table where we have to figure out either h plus and concentration or which my nasa and concentration or…. #1 relatively easy, no ice table required because eq’m concentrations are given for the reaction ch4(g) h2o(g) ↔ co(g) 3h2 (g) @ 1500° c an equilibrium mixture of these gases was found to have the following concentrations [co] = 0.300m, [h2] = 0.800m and [ch4] = 0.400m. kc @ 1500° c = 5.67. determine the equilibrium concentration of h2o in this mixture. A solution is called acidic if the concentration of hydronium is larger than the concentration of hydroxide while a solution is called basic if the concentration of hydroxide is larger than the concentration of hydronium. this corresponds to ph values of less than seven for acidic solutions and ph values of greater than seven for basic solutions.

Solved Complete The Following Table All Solutions Are At 25 Chegg #1 relatively easy, no ice table required because eq’m concentrations are given for the reaction ch4(g) h2o(g) ↔ co(g) 3h2 (g) @ 1500° c an equilibrium mixture of these gases was found to have the following concentrations [co] = 0.300m, [h2] = 0.800m and [ch4] = 0.400m. kc @ 1500° c = 5.67. determine the equilibrium concentration of h2o in this mixture. A solution is called acidic if the concentration of hydronium is larger than the concentration of hydroxide while a solution is called basic if the concentration of hydroxide is larger than the concentration of hydronium. this corresponds to ph values of less than seven for acidic solutions and ph values of greater than seven for basic solutions. To complete the table, we need to understand how to calculate the [oh^ ], ph, and identify if the solution is acidic or basic based on the [h 3o^ ] concentration. Question: complete the following table. (all solutions are at 25 ∘c.) complete the first column. complete the 2nd column. complete the 3rd column. complete the 4th column. to two sig figs. [h3o ] [oh−] ph acidic or.
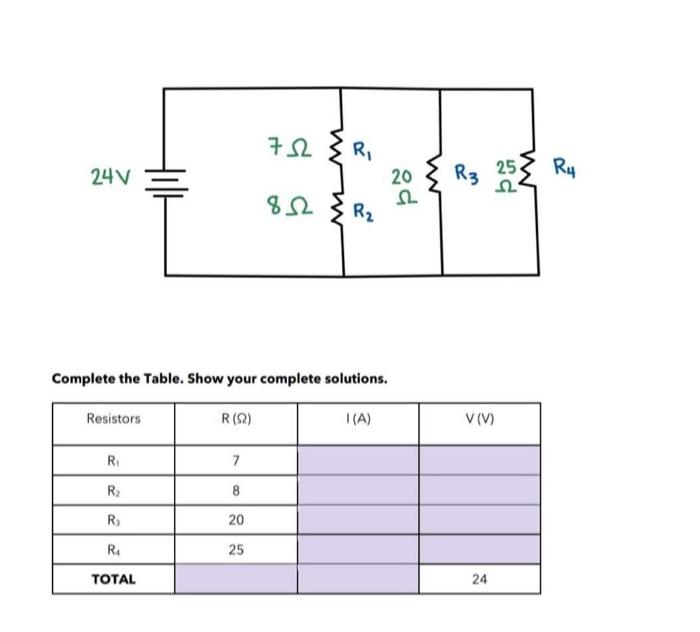
Solved Complete The Table Show Your Complete Solutions Chegg To complete the table, we need to understand how to calculate the [oh^ ], ph, and identify if the solution is acidic or basic based on the [h 3o^ ] concentration. Question: complete the following table. (all solutions are at 25 ∘c.) complete the first column. complete the 2nd column. complete the 3rd column. complete the 4th column. to two sig figs. [h3o ] [oh−] ph acidic or.
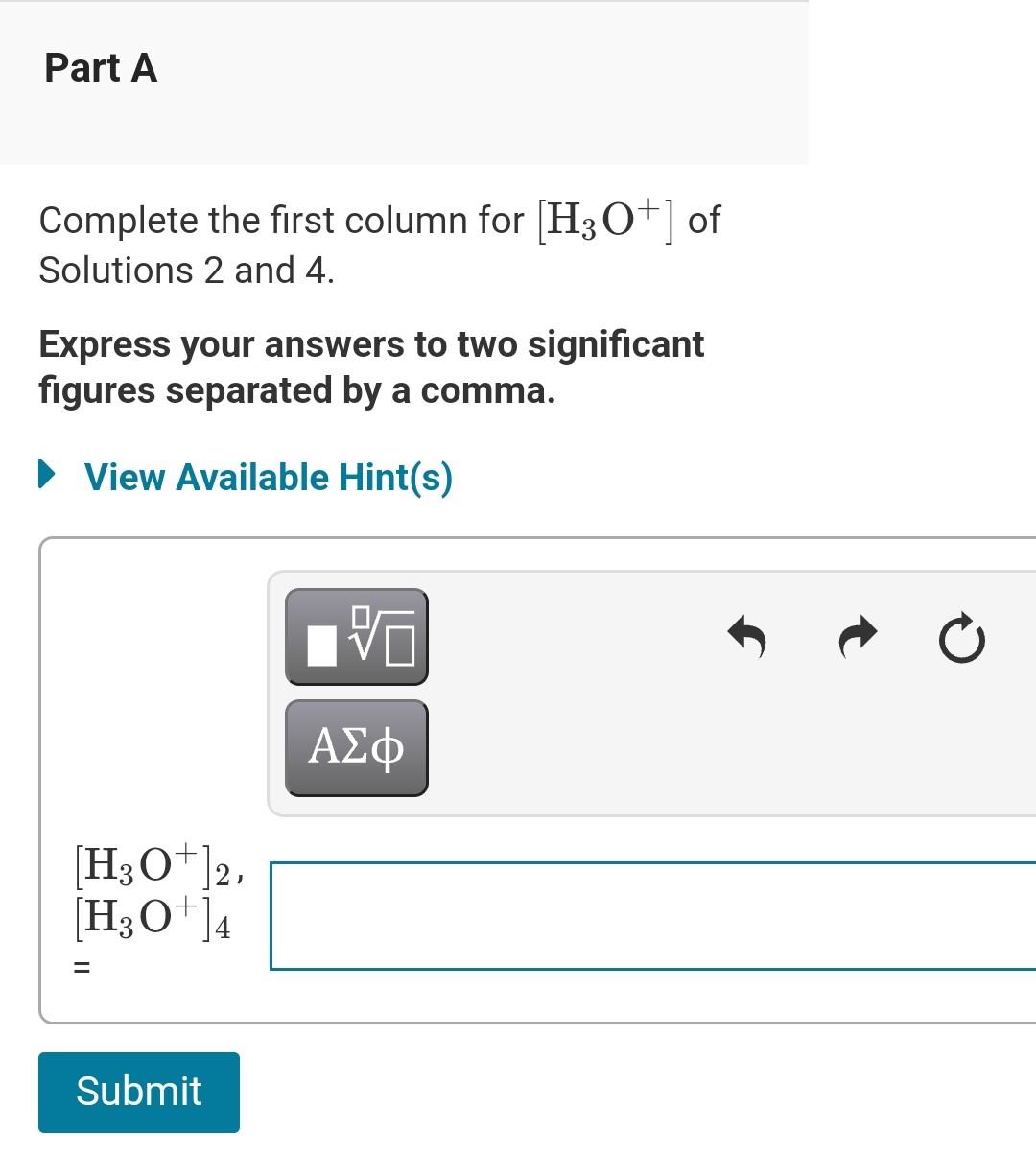
Solved Complete The Following Table All Solutions Are At Chegg
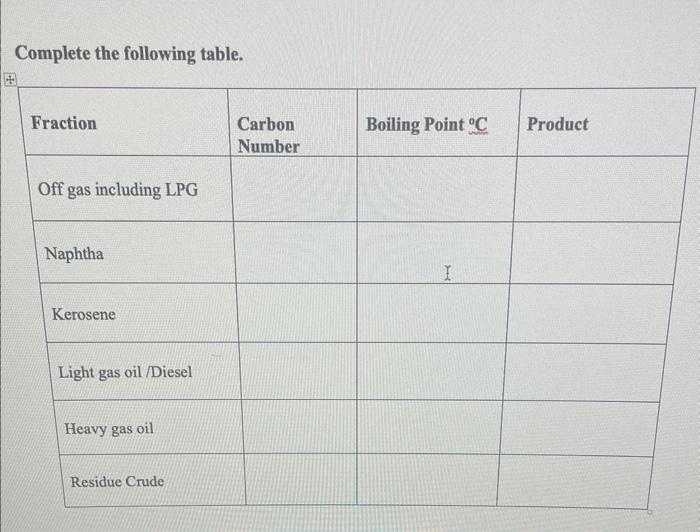
Solved Complete The Following Table Chegg
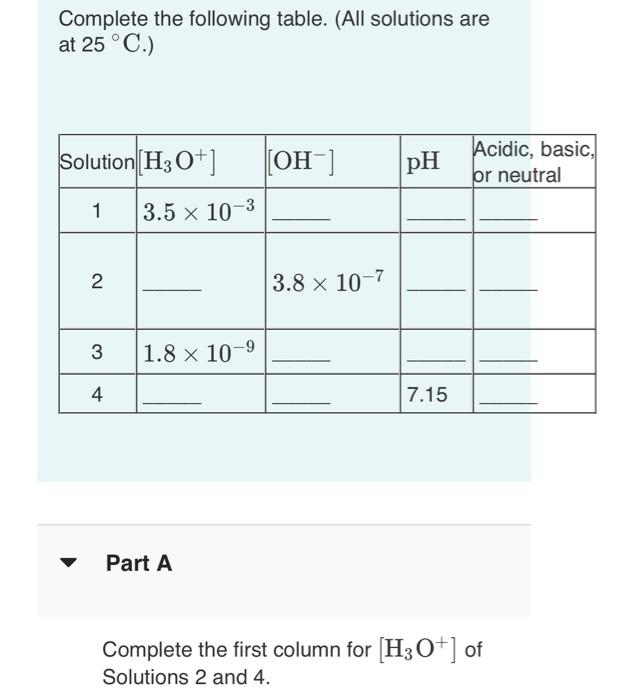
Solved Complete The Following Table All Solutions Are At Chegg