
Biological Classification Pdf Guidelines for national authorities on quality assurance for biological products, annex 2, trs no 822 18 october 2015 guidelines on procedures and data requirements for changes to approved vaccines, annex 4, trs no 993 guidelines on procedures and data requirements for changes to approved vaccines, annex 4, trs no 993. Biological therapeutics, also referred to as biologicals, are those class of medicines which are grown and then purified from large scale cell cultures of bacteria or yeast, or plant or animal cells. biologicals are a diverse group of medicines which includes vaccines, growth factors, immune modulators, monoclonal antibodies, as well as.

2 Biological Classification Pdf The united nations agency working to promote health, keep the world safe and serve the vulnerable. This guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and biological drug quantification in biological matrices and their application in the analysis of study samples. adherence to the principles presented in this guideline will ensure the quality and consistency of the bioanalytical data in support of the development and market approval. Overview . biotherapeutic products are an increasingly important component of global health care. several who guidelines on the evaluation of biotherapeutic products have been produced that provide a set of principles on the regulatory evaluation of such products. Who bs 2024.2471 – who 1st international reference panel for adventitious virus detection in biological products by high throughput sequencing (hts) technologies who bs 2024.2472 – new who reference material projects.

Biological Databases Classification Overview . biotherapeutic products are an increasingly important component of global health care. several who guidelines on the evaluation of biotherapeutic products have been produced that provide a set of principles on the regulatory evaluation of such products. Who bs 2024.2471 – who 1st international reference panel for adventitious virus detection in biological products by high throughput sequencing (hts) technologies who bs 2024.2472 – new who reference material projects. The catalogue of international reference standards for biological products is updated following the expert committee on biological standardization meetings. see below for the catalogue, listed in alphabetical order and includes additional information. alphabetical list. Biotechnology describes biological processes that have been manipulated or modified in some way through modern science. a major industrial application of biotechnology is in the development and preparation of biological medicinal products using genetically engineered bacteria, yeast, fungi, cells or even whole animals and plants.

Biological Databases Classification The catalogue of international reference standards for biological products is updated following the expert committee on biological standardization meetings. see below for the catalogue, listed in alphabetical order and includes additional information. alphabetical list. Biotechnology describes biological processes that have been manipulated or modified in some way through modern science. a major industrial application of biotechnology is in the development and preparation of biological medicinal products using genetically engineered bacteria, yeast, fungi, cells or even whole animals and plants.

Pdf Classification Identification On Biological Databases
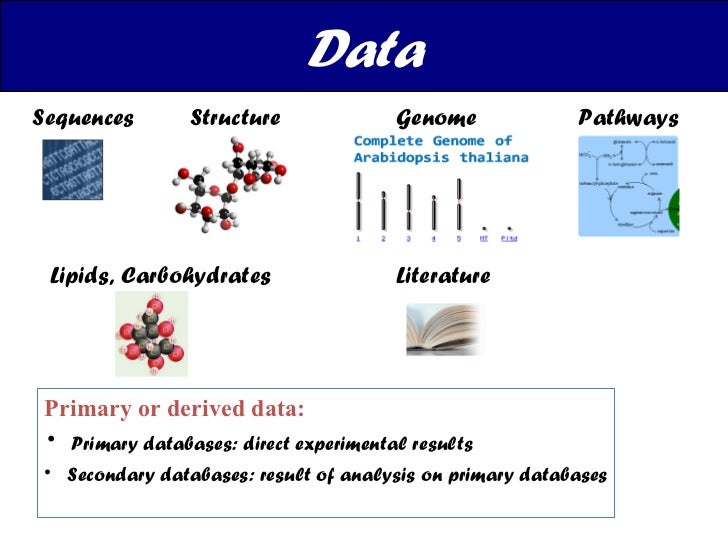
Biological Databases