
Enthalpy Vs Entropy Pdf Enthalpy Entropy An enthalpy change describes the change in enthalpy observed in the constituents of a thermodynamic system when undergoing a transformation or chemical reaction. Enthalpy is a state function of a thermodynamic system and depends on other state functions. mathematically, it is the sum of the internal energy and the product of the pressure and volume of the system.

Enthalpy And Entropy Pdf Entropy Enthalpy Enthalpy is an energy like property or state function—it has the dimensions of energy (and is thus measured in units of joules or ergs), and its value is determined entirely by the temperature, pressure, and composition of the system and not by its history. In thermodynamics, the enthalpy is the measure of energy in a thermodynamic system. it is the thermodynamic quantity equivalent to the total heat content of a system. the enthalpy is defined to be the sum of the internal energy e plus the product of the pressure p and volume v. Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume (pv p v) given by the equation: h = u pv (1) (1) h = u p v. when a process occurs at constant pressure, the heat evolved (either released or absorbed) is equal to the change in enthalpy. The enthalpy change of a reaction depends on the physical states of the reactants and products, so these must be shown. for example, when 1 mole of hydrogen gas and mole of oxygen gas change to 1 mole of liquid water at the same temperature and pressure, 286 kj of heat are released. if gaseous water forms, only 242 kj of heat are released.

Enthalpy Vs Entropy Know The Difference Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume (pv p v) given by the equation: h = u pv (1) (1) h = u p v. when a process occurs at constant pressure, the heat evolved (either released or absorbed) is equal to the change in enthalpy. The enthalpy change of a reaction depends on the physical states of the reactants and products, so these must be shown. for example, when 1 mole of hydrogen gas and mole of oxygen gas change to 1 mole of liquid water at the same temperature and pressure, 286 kj of heat are released. if gaseous water forms, only 242 kj of heat are released. Enthalpy is the measurement of energy in a thermodynamic system. the quantity of enthalpy equals to the total content of heat of a system, equivalent to the system’s internal energy plus the product of volume and pressure. Learn what enthalpy is, its formula, significance in thermodynamics, and real world applications in engineering and science.
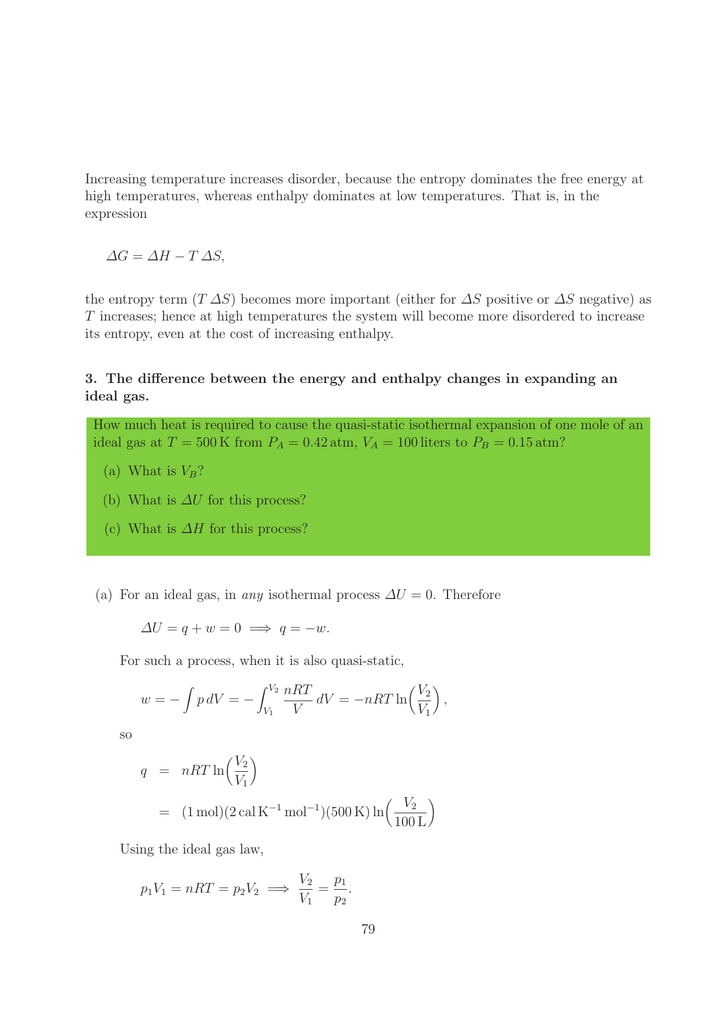
Enthalpy Vs Entropy Maingolf Enthalpy is the measurement of energy in a thermodynamic system. the quantity of enthalpy equals to the total content of heat of a system, equivalent to the system’s internal energy plus the product of volume and pressure. Learn what enthalpy is, its formula, significance in thermodynamics, and real world applications in engineering and science.
Enthalpy Vs Entropy The Science Behind Energy And Disorder
Enthalpy Vs Entropy What S The Difference