
33 Strong Vs Weak Acids S Pdf Acid Electrolyte Main difference – strong vs weak acids an acid is a molecule or other species which can donate a proton or accept an electron pair in reactions. acids are classified into two groups known as strong acids and weak acids. the main difference between strong and weak acids is that strong acids dissociate completely in aqueous solutions whereas weak acids partially dissociate in aqueous solutions. In the world of chemistry, understanding the difference between strong acids and weak acids is fundamental for both students and professionals alike. strong acids are known for their ability to completely dissociate in water, making them a pivotal topic in chemical reactions and laboratory experiments.

Understanding Strong And Weak Acids Acids are classified as either strong or weak, based on their ionization in water. a strong acid is an acid which is completely ionized in an aqueous solution. The number of protons given off by a particular acid actually determines the strength of the acid – whether it is a strong acid or a weak acid. in order to understand the strength of the acids, one need to compare their tendency to donate protons to the similar base (mostly water). Strong acid vs. weak acid what's the difference? strong acids and weak acids are both types of acids, but they differ in their ability to dissociate in water. strong acids completely dissociate into ions when dissolved in water, resulting in a high concentration of hydrogen ions (h ). Strong acid weak acid let's discuss them one by one what is strong acid? strong acids are those acid that dissociates completely in aqueous solution. they dissociate completely in cation and anion when dissolved in aqueous solution. they loose h ion very quickly in an aqueous solution. strong acid has a lower ph because of its ability to lose all protons. strong acids are good conductors of.

Strong Acids Vs Weak Acids What S The Difference Strong acid vs. weak acid what's the difference? strong acids and weak acids are both types of acids, but they differ in their ability to dissociate in water. strong acids completely dissociate into ions when dissolved in water, resulting in a high concentration of hydrogen ions (h ). Strong acid weak acid let's discuss them one by one what is strong acid? strong acids are those acid that dissociates completely in aqueous solution. they dissociate completely in cation and anion when dissolved in aqueous solution. they loose h ion very quickly in an aqueous solution. strong acid has a lower ph because of its ability to lose all protons. strong acids are good conductors of. Ph scale and indicators weak acids vs strong acids [gcse chemistry only] many chemicals can be classified as being acidic, neutral or alkaline by using indicators. The behavior of strong acids and weak acids also varies in different chemical reactions. for instance, strong acids can readily neutralize bases, producing salt and water.

Ppt Strong Acids Vs Weak Acids Powerpoint Presentation Id 2362533 Ph scale and indicators weak acids vs strong acids [gcse chemistry only] many chemicals can be classified as being acidic, neutral or alkaline by using indicators. The behavior of strong acids and weak acids also varies in different chemical reactions. for instance, strong acids can readily neutralize bases, producing salt and water.
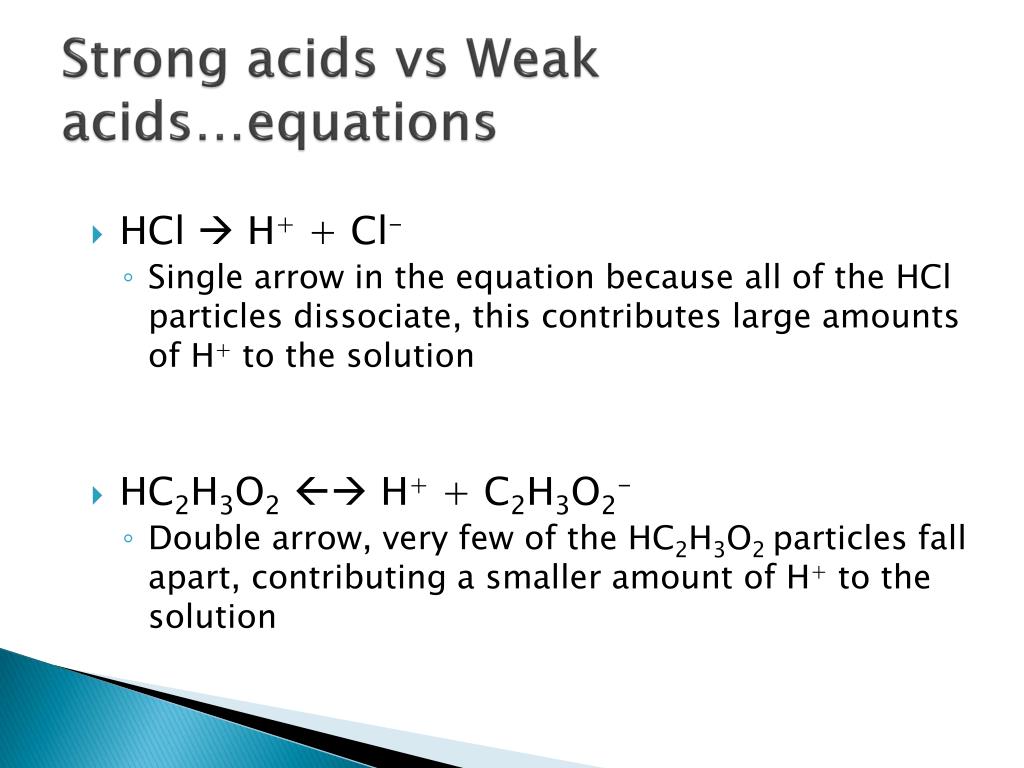
Ppt Strong Acids Vs Weak Acids Powerpoint Presentation Free Download
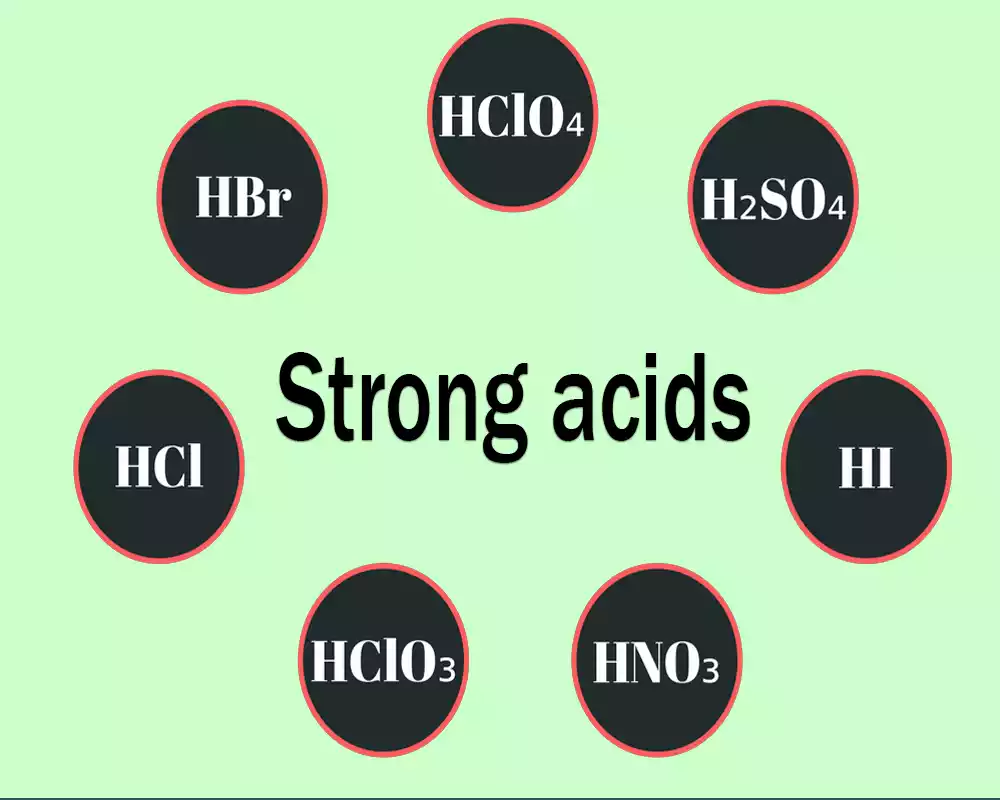
Difference Between Weak Acids And Strong Acids Productive Difference